EKG Device
The Challenge
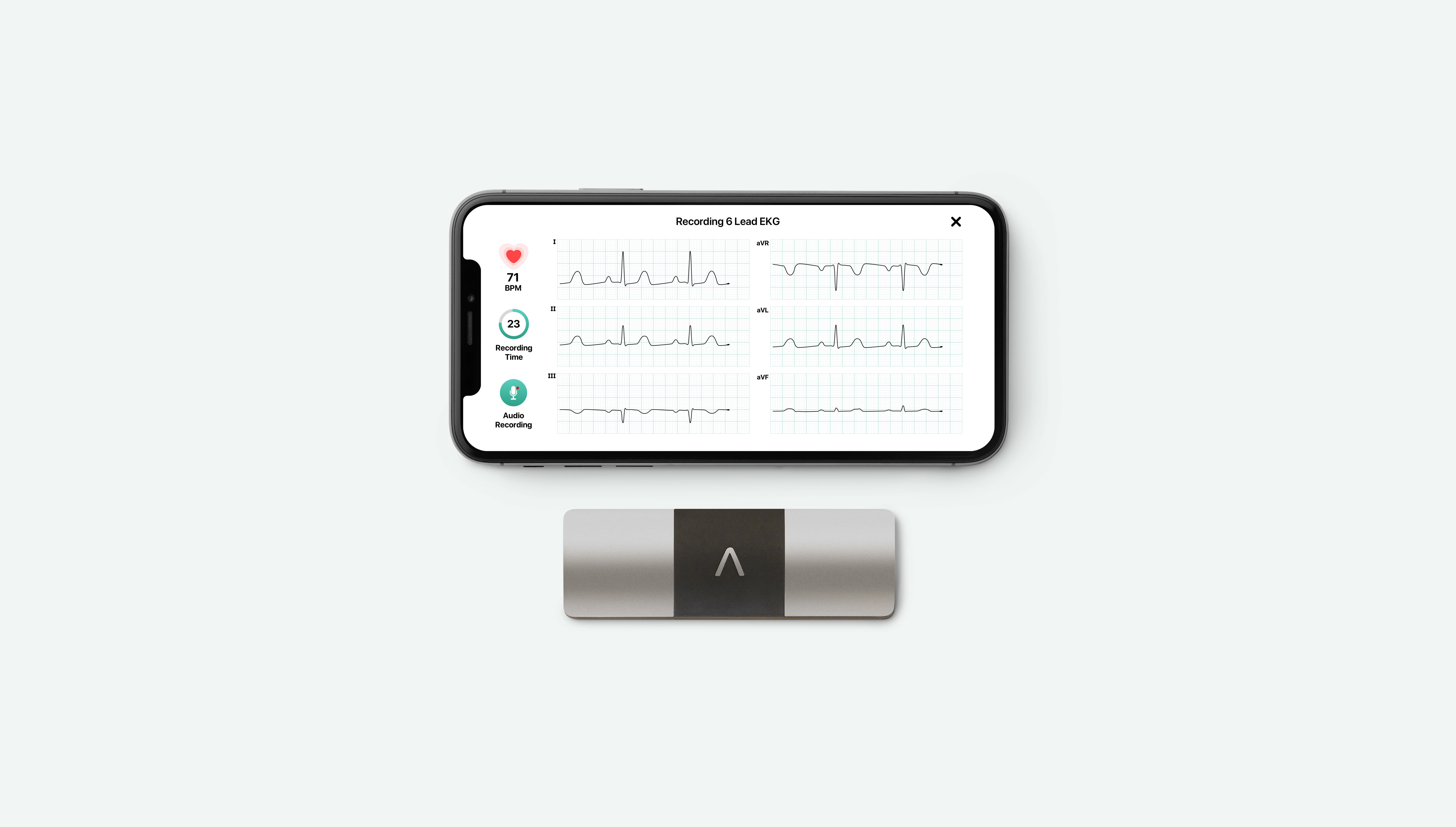
As the first cost-effective, non-invasive, smartphone brand-agnostic solution that achieves 6-lead EKG recordings, the product needed to instill patient confidence in the product’s accuracy, reliability and effectiveness. The product design also had to be manufacturable at scale, using the highest quality materials and components, while meeting globally recognized (including FDA and FCC) standards.
PCH Solution
At the early stages of the project, PCH and our customer consulted with PCH’s manufacturing engineering team regarding sourcing, tooling, and assembly requirements. PCH’s guidance regarding the manufacturing feasibility and risks associated with each design option was crucial in choosing the design direction, enabling our customer to choose an optimal design while being certain that it could be engineered and manufactured at scale. Once the design options were narrowed, PCH began prototyping the device.
PCH engineers built, iterated on and tested the primary design concept at PCH’s state-of-the-art prototyping labs in San Francisco. Functional builds were made to understand and test the primary industrial design concept, assembly process, total part-count, features, functionality, and reliability.
The Result
Our customer recognized the value of PCH’s in-depth knowledge and deep experience in engineering, product development, manufacturing at the earliest product concept phase. This close working relationship proved essential in not only delivering our customer’s product vision and robust technical and user experience requirements, but also in creating a beautiful high-quality product that meets global standards and cost target for a consumer product.